Newsroom

St. Luke's Begins Ground-Breaking Cancer Treatment
St. Luke’s is the first community hospital in the region to offer a ground-breaking, outpatient cancer treatment-bispecific antibodies (bsAbs)!
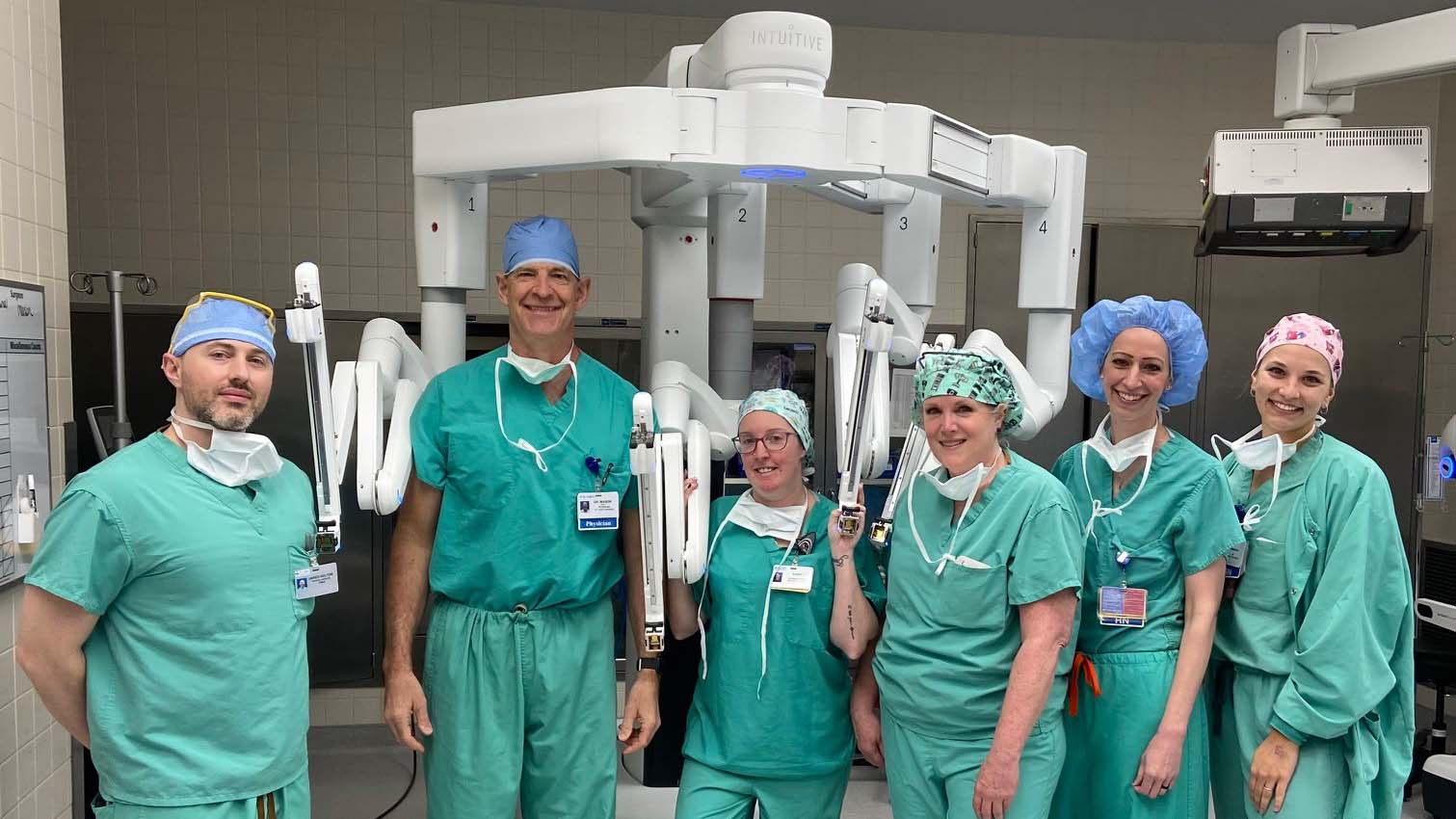
St. Luke’s Becomes the First Hospital in the Region to Implement High-Tech Surgical System
The installation of the da Vinci 5® robotic system expands St. Luke’s surgical capabilities, supporting advanced, minimally invasive care and enhanced recovery.

St. Luke’s Named One of The Best Hospitals in The State of Missouri, and a Best Regional Hospital, St. Louis Metro
St. Luke’s has again been recognized as a 2024-2025 Best Hospital in 14 categories by U.S. News & World Report. In addition to ranking 5th in Missouri and 4th in the St. Louis Metro area in Best Regional Hospitals, St. Luke’s received “High Performing” ratings in 13 Frequently Treated Procedures & Conditions.

Newsweek™ Names St. Luke’s To “America’s Best Cancer Hospitals, 2024” List For The 2nd Year In A Row!
St. Luke’s is thrilled to announce that its Center for Cancer Care has been recognized as one of Newsweek's "America's Best Cancer Hospitals 2024."

St. Luke’s Hospital Named to the Forbes List of America’s Best Employers for Women
St. Luke’s is honored to be named to Forbes list of America’s Best Employers for Women 2024! This prestigious award is presented by Forbes and Statista Inc. The award list can be viewed on the Forbes website.

St. Luke's Hospital Named Among the Top 5% in the Nation
St. Luke’s Hospital announced today that it has achieved the Healthgrades 2024 Outstanding Patient Experience Award™ and is the only hospital in Missouri to achieve the Outstanding Patient Experience Award for 14 years in a row (2011-2024).

St. Luke’s Wins the Trifecta at the 41st Annual Healthcare Advertising Awards!
St. Luke’s is proud to be a multi-category winner in the largest and most well recognized healthcare advertising awards competition in the nation!

St. Luke’s Surgeon Performs World’s First Transcarotid Neuroprotection System PLUS Procedure
St. Luke’s is thrilled to announce that Brian Peterson, MD, FACS, FSVS, vascular surgeon at St. Luke’s Heart and Vascular Institute, performed the world’s first, next generation, Trans Carotid Artery Revascularization.

St. Luke's Hospital is Honored in Becker’s Hospital Review
St. Luke’s is thrilled to be named to the Becker's Hospital Review list of the “Hospitals and health systems with great diabetes and endocrinology programs."

St. Luke’s Surgeon Hosts Delegation from China for Trans Carotid Artery Revascularization Training
Brian Peterson, MD, FACS, FSVS, vascular surgeon at St. Luke’s Heart and Vascular Institute, welcomed a medical delegation from China today in advance of the TCAR procedure’s debut in Asia next month.
Media Contact
Marketing/PR